Drug Formulation
Early identification of the optimal drug formulation to deliver your active pharmaceutical ingredient (API) with an optimal release profile in the right compartment of the body is crucial to increase the likelihood of success for your drug development program.
At Bioneer, we have in-depth scientific expertise with most aspects of drug formulation and pharmaceutical development and we have successfully developed a broad range of dosage forms for biotech and pharmaceutical companies over the past few decades covering small molecule, peptide and protein modalities.
We perform advanced as well as more conventional in vitro modelling to understand and simulate the behaviour of drug products in clinical settings. Our unique approach to gastro-intestinal modelling using a proprietary Dynamic Gastric Model (DGM) has predicted and delivered oral dosage form solutions for innovator drugs and for generics alike.
For small molecules, our primary focus is on Biopharmaceutical Classification System Class 2 and 4 drug molecules. We specialize in developing amorphous solid dispersions and lipid-based drug delivery systems to enhance absorption.

For peptides and proteins, we focus on developing stable injectable formulations while also exploring oral dosage forms for peptides. The stability of these formulations is rigorously assessed using advanced analytical techniques.
Our service packages are tailored to each unique project, ensuring a highly flexible and adaptable development process. As your R&D partner, we strive to mitigate potential challenges and sudden changes in your timelines or priorities. Our dynamic and versatile approach to pharmaceutical innovation allows us to embrace a wide array of projects, always driven to develop new methods or specialized services as needed.
The Dynamic Gastric Model (DGM)
Bioneer is a center of excellence for gastro-intestinal (GI) modelling. Utilizing a unique Dynamic Gastric Model (DGM) and Intestinal Digestion Module, we help you generate unparalleled insights into:
- Biorelevant in vitro simulation of the human GI tract
- Prediction of dosage form and drug behavior
Applicable for a broad range of applications:
- Tablet/capsule gastric behavior (float /sink, disintegration time, etc.)
- Bioequivalence studies (e.g. generics)
- Drug release from modified release systems
- Gastric integrity of matrix dosage forms (e.g. dose dumping)
- Gastric integrity of enteric-coated dosage forms
- Food-effect for immediate & modified release dosage forms
- Gastric integrity of gastro-retentive systems
- Simulation of special populations (e.g. infants or patients)
- Drug dissolution, supersaturation, precipitation
The DGM is the only in vitro model that accurately simulates the complex dynamic biochemical composition and peristalsis of the human stomach. It gives key insights into the behavior of oral dosage forms and how your drug is released/dissolved under simulated gastro-intestinal conditions.
The DGM incorporates the body/fundus and the antrum of the human stomach. In these compartments, the storage and digestion of liquid and/or food is simulated, along with the peristaltic movements. The antral peristalsis is achieved through hydrodynamics (rather than mechanical stress), which simulates the sheer forces oral dosage forms will encounter in the GI tract. Furthermore, the emptying rate, pH profile and dynamic fluid composition represents that of the human stomach. Like no other available GI model, the DGM is able to accommodate and digest an entire meal such as the high-fat and high-calorie meal, which is recommended by the FDA to evaluate food effects (“FDA breakfast”). Thus, it allows for both fasted and fed-state condition simulations, and the study protocol may be adjusted to mimic that of a clinical study.
With the DGM and Intestinal Digestion Module we generate accurate drug dissolution data that we convolute into clinical pharmaco-kinetic profile predictions to achieve In vitro-In vivo Correlation (IVIVC) for your drug. The set-up includes simulating gastric emptying of the drug or dosage form from the stomach to the intestine, secretion of bile and pancreatic enzymes and drug dissolution in the intestine. It enables us to assess how different factors such as pH, bile, enzymes, transit time, fluid volume and food ingestion influence drug dissolution.
Biorelevant in vitro models
At Bioneer, we leverage state-of-the-art technologies to study drug permeation, dissolution, and behavior of oral dosage forms to optimize absorption, bioavailability and release of your drug.
Our advanced systems, provide precise and physiologically relevant assays. These tools are essential for optimizing drug formulations, delivery systems, and enhancing therapeutic efficacy. Whether your focus is ocular, buccal, intestinal, or topical administration, our tailored assays and innovative approaches ensure accurate and reliable data to support your drug development needs.
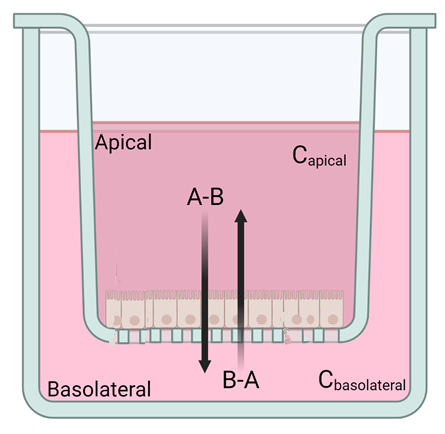
Permeation models
Selected methodologies:
- MicroFlux
- Franz Diffusion cells
- Transwell
Our permeation models are tailored to predict in vivo performance of the specific formulation principle of interest. We utilize commercially available and in-house developed artificial membranes for investigating passive diffusion, while we for active transport utilize cell-based or tissue barriers. Depending on the route of administration, we decide on the best suitable setup, e.g. MicroFLUX™, Franz diffusion cells or Transwells™, in combination with the most physiologically relevant media towards establishing IVIVC.
Dissolution models
Selected Methodologies:
- MicroDiss
- Lipolysis
- Intestinal Dissolution Module
.
We offer advanced dissolution services to ensure optimal bioavailability and therapeutic efficacy for small molecule drugs. Utilizing techniques such as MicroDISS for precise small-scale testing and lipolysis for simulating digestive processes, we provide comprehensive insights into drug dissolution behavior. Our services also include various USP apparatus models and biorelevant dissolution testing, such as intestinal simulation, to meet diverse formulation needs.
Cellular Barrier Models
Cellular barrier models are crucial in early drug development for screening drug compounds by replicating the complexities of human biological barriers. These in vitro models are instrumental for assessing a drug’s permeability across specific barriers, determining whether it crosses via passive diffusion or active transport. In addition, we can screen for potential drug interactions with transport proteins to identify if a drug is a substrate or inhibitor. This data is vital for predicting a drug’s efficacy, safety, and pharmacokinetics before clinical trials.
Bioneer services comprise various cellular barrier models, including our state-of-the-art blood-brain barrier model created from human induced pluripotent stem cell lines differentiated into brain microvascular endothelial-like cells. We also utilize established immortalized cell lines for intestinal and kidney epithelium models. Additionally, we offer in vivo models of biological membranes, such as dermal, buccal, and intestinal tissues. These models are robust platforms for characterizing drug compounds and evaluating different formulations, thus accelerating drug development and reducing the need for animal testing.
Blood Brain Barrier
To model the human blood-brain barrier, we use our in-house human induced pluripotent stem cell lines, which can be differentiated towards brain microvascular endothelial cells, which is the cell type of the neurovascular unit, which mainly contributes to the blood-brain barrier function.
In addition to our original human induced pluripotent stem cell line (BIONi010-C) we can also offer services with a similar cell line (BIONi010-C-48), which is the original cell line transfected with the human MDR1 gene to express the human efflux transporter P-glycoprotein. If your project requires a blood-brain barrier model expressing a specific target protein, we are open to discuss possibilities for gene editing work to generate a human induced pluripotent stem cell line customized to your needs.
Intestinal Epithelial Barrier
We use immortalized intestinal epithelial cells (e.g. Caco-2 cells) to accurately replicate the complex intestinal epithelium, providing a versatile platform for studying drug compound permeability and interactions with transport proteins in the intestinal barrier. With options available for models both with and without mucus secretion, we can assess how drug compounds interact with the intestinal barrier. The Caco-2 cell line is also used as an industry-standard for assessing a general human cell permeability and interactions with transport proteins.
Renal Epithelial Barrier
With monolayers of the immortalized cell lines MDCK II and MDCK II hMDR1 we can assess the drug compound permeability across the renal epithelium, as well as interactions with efflux transport proteins expressed by the cell monolayers. In addition, the MDCK II cell lines are often used as an industry standard to assess a general cellular permeability and interactions with transport proteins.
Engineered Cell Lines
We use engineered porcine epithelial cell lines (based on the IPEC-J2 cell line) transfected with either the human MDR1 gene or the rat mdr1a gene to express human P-glycoprotein or rat P-glycoprotein. These cell lines are unique due to the fact that they are extremely tight barrier models combined with an expression of endogenous P-glycoprotein that is negligible compared to the expression of either human P-glycoprotein or rat P-glycoprotein. In this way, these cell lines are tailored to study specific interactions with either human or rat P-glycoprotein without contribution from endogenous P-glycoprotein as is the case with the MDCK II cells.
Peptides & Proteins
At Bioneer, we specialize in providing peptide and protein pre-formulation and characterization services to meet your specific needs in relation to pharmaceutical development.
We offer comprehensive analytical packages utilizing a wide array of state-of-the art instrumentation. We have all needed equipment to develop coated tablets and liquid filled capsules.
From initial screening of peptide and protein compounds to pre-formulation activities of lead candidates, we support you throughout the entire process. Projects can be divided into smaller work packages, which can be adjusted as your project progresses.
Development of physically and chemically stable peptide and protein formulations involves comprehensive solubility and stability screening studies. This we typically achieve by applying a rational and iterative approach where the pH, concentration, excipients, and buffer systems are evaluated to ensure high solubility and sufficient shelf-life of your drug candidate to meet its target product profile.
Solubility
Solubility studies, including pH titrations, offer valuable insight into viable concentration and pH ranges. Depending on the tolerance toward ionic strength of your molecule, we test both ionic or non-ionic tonicity modifiers to ultimately ensure a final formulation that meets the pH and tonicity requirements.
Pre-formulation experiments and continuous evaluation of the results lead to an ongoing assessment of whether a peptide or protein can be effectively formulated into a drug product as desired. For instance, pre-formulation findings might indicate the need for a lyophilized or a liquid-frozen over a liquid formulation, prompting parallel development of several formulation strategies to ensure a fallback option. In designing pre-formulation studies, we prioritize efficiency and minimal material consumption and select appropriate analytical techniques and stress conditions to maximize information gain.
Stability
Chemical and physical stability studies alongside forced degradation assays are pivotal in pinpointing specific excipients with either a stabilizing or destabilizing effect. This evaluation involves analyzing peptide and protein formulations with regard to physical and chemical degradation, ensuring the development of robust, simple, and effective formulations.
Physical stability
Selected methodologies:
- ThT (Fibrillation) Assay
- SAXS/DLS
- Circular Dichroism
- Physical Stress Test
An efficient way to discriminate between formulations from a physical stability stand point is through fibrillation and aggregation assays. We use the selective binding of Thioflavin T (ThT) to beta-sheet rich structures in fibrils, leading to an increase in red-shifted fluorescence emission signal. By tracking this fluorescence signal over time, we assess the fibrillation tendency of peptide or protein formulations and rank them based on the fibrillation lag-time. The ThT assay can be conducted under different conditions, including agitation and elevated temperatures, to provide comprehensive insights into peptide or protein stability.
Rotational stress or excessive shaking can simulate different conditions encountered during manufacturing and transportation or serve to evaluate the resilience of different formulations to physical stress related degradation. Concurrently, subjecting the formulations to multiple freeze-thaw cycles can highlight the potential benefits of incorporating surfactants for enhanced physical stability. Additionally, characterizing aggregates or multimers can be achieved through dynamic light scattering (DLS), size exclusion chromatography (SE-HPLC), or small angle scattering (SAXS). The latter can be carried out either in-house or at a large-scale facility (synchrotron). Circular dichroism (CD) allows for the monitoring of conformational changes in both the secondary and tertiary structure. By subjecting peptides or proteins to temperature ramps CD spectroscopy facilitates the evaluation of transition temperatures and thermally induced conformational states. These types of experiments provide important information into optimal storage conditions, in particular for highly temperature-sensitive biomolecules
Chemical stability
Selected methodologies:
- Forced Degradation
- Deamidation
- Oxidation
- Reduction of disulphide bridges
Chemical stability can be assessed by forced degradation assays involving thermal, oxidative, reducing, strongly acidic, or alkaline stress conditions among others. Depending on factors such as the sequence, secondary and tertiary structure, and the presence of certain problematic (exposed) amino acids, peptides or proteins may be susceptible to e.g. oxidation, deamidation, cyclic imide formation, or disulfide bridge breakage. These degradation patterns can be analyzed by HPLC-UV and LC-MS. By identifying certain degradation peaks and monitoring their progression over time, particularly at elevated temperatures, we gain valuable insights into formulation requirements. Forced degradation studies and temperature stability assessments aid in determining optimal storage conditions ensuring formulation integrity and efficacy throughout the product’s shelf-life. This holistic approach enables us to develop robust formulations capable of withstanding environmental stressors.
Freeze-dried Formulation Development
For sensitive peptides and proteins requiring long-term stability or storage under stressful conditions such as shaking or elevated temperatures, freeze-drying often offers the best solution for successful formulation development. We offer freeze-drying formulation development tailored to each specific project and needs and offer small-scale technical batch production of 300-600 vials depending on vial size. The lyophilization process involves three main stages: freezing, primary drying (sublimation), and secondary drying (desorption). During freezing, the product is cooled to convert the water into ice. In the primary drying phase, ice is removed through sublimation under low pressure, while secondary drying eliminates residual moisture by desorption, ensuring product stability. The vials are closed under vacuum and crimped for optimal stability during storage.
We focus on evaluating the physical and chemical stability of the freeze-dried product both by assessing the stability and structural integrity of the freeze-dried cake and by testing the chemical and physical stability of the reconstituted formulation.
For sensitive peptides and proteins requiring long-term stability or storage under stressful conditions such as shaking or elevated temperatures, freeze-drying often offers the best solution for successful formulation development. We offer freeze-drying formulation development tailored to each specific project and needs and offer small-scale technical batch production of 300-600 vials depending on vial size. The lyophilization process involves three main stages: freezing, primary drying (sublimation), and secondary drying (desorption). During freezing, the product is cooled to convert the water into ice. In the primary drying phase, ice is removed through sublimation under low pressure, while secondary drying eliminates residual moisture by desorption, ensuring product stability. The vials are closed under vacuum and crimped for optimal stability during storage.
We focus on evaluating the physical and chemical stability of the freeze-dried product both by assessing the stability and structural integrity of the freeze-dried cake and by testing the chemical and physical stability of the reconstituted formulation.
Final Formulation
In-use studies
We conduct in-use studies to assess the compatibility of formulations with materials used in clinical settings. These studies involve procuring the exact materials used for administration and handling in the clinic and carry out experiments simulating the clinical trial setup, regardless of the administration route. Through this approach, we can simulate various scenarios, such as single or multiple ascending dose experiments, mimicking dosing volume, infusion time etc. This allows us to evaluate the stability of the drug product when in contact with in-use materials and its adsorption to these surfaces. Assessing formulation performance under real-world conditions, including varying temperatures, light exposure, and physical handling, is crucial in peptide and protein formulation development, ensuring stability, efficacy, and safety throughout the product’s lifecycle.
Technical batch production
We offer small scale non-GMP technical batch productions of peptides and proteins, e.g. production of reference materials or material for pre-clinical tox studies. We offer this in various formats, including liquid, liquid-frozen, and freeze-dried. While our technical batch production services adhere to rigorous quality standards, it is important to note that they are not GMP-certified. This approach enables us to provide rapid and cost-effective production cycles without compromising on quality. Trust our experienced team to deliver consistent, high-quality results tailored to your specific requirements.
Small Molecules
At Bioneer, we offer comprehensive services for the development of small molecule drugs covering every aspect from pre-formulation to product development. Based on physico-chemical characterization of your drug and the pharmacokinetic profile desired, we help you determine the best formulation strategy.
We offer comprehensive analytical packages utilizing a wide array of state-of-the art instrumentation. We have all needed equipment to develop coated tablets and liquid filled capsules.
Formulation Development
Selected Methodologies:
- Lipid based drug delivery systems (SNEDDS)
- Mesoporous Silica formulations
- Controlled Release
We specialize in enabling formulations for poorly soluble drugs (BCS Class 2 and 4), to increase solubility, dissolution rate and ultimately drug absorption. Our expertise includes development of lipid-based formulations (e.g. self-nanoemulsifying drug delivery systems (SNEDDS)), Amorphous Solid Dispersions (ASD) and mesoporous silica–based formulations, focusing on high drug load and fast release.
In addition, we also have expertise in developing controlled release formulations for sustained drug release over extended periods.
Characterization
Selected Methodologies:
- X-ray powder diffraction
- Thermal Analysis
- Intrinsic Dissolution Testing
- Amorphous Dispersion
Drug characterization is crucial as it provides a deep understanding of the physical, chemical, and biological properties of a compound. We employ techniques such as X-ray Powder Diffraction (XRPD) to analyze the crystalline structure of drug molecules, while Thermal Analysis (TGA DSC) evaluates drug stability and melting points. Microscopy (PLM, SEM) visualizes drug particles and assesses morphology. Solubility Studies, Intrinsic Dissolution Testing, and Amorphous Dispersion Screening further contribute to understanding dissolution behavior and enhancing drug performance


