Transient Gene Expression
Transient Gene Expression (TGE) is the optimal choice for achieving rapid mammalian production, serving as the initial stage in assessing the expression of proteins in mammalian cells. Within a timeframe as short as 5 weeks, we can provide protein for research purposes and concurrently evaluate up to 72 constructs to ascertain the feasibility of protein expression in HEK293 or CHO cells. Upon the completion of initial feasibility studies, we can scale expression up to 30 L culture volume using shake-flasks.
- Systems: HEK293-6E, CHO-3E7, or Your Preferred System
- Scale from 1mL to 30L
- Test up to 72 constructs in parallel
- As little as 5 weeks to purified protein
- Yields up to 500 mg/L
Workflow at Bioneer
Leveraging our expertise across diverse protein types, we provide early insights into the expression of your protein and mitigate risk through a flexible step-wise project approach with Go/No-Go decision points.
Process Step |
Our approach |
Time |
|---|---|---|
|
Gene Design and Plasmid Preparation
|
|
2 – 4 weeks
|
|
Feasibility Studies & Construct Screening
|
|
2 – 3 weeks
|
Report Outlining Expression Results (Go/No Go) |
||
|
Upscaled Expression
|
|
2 – 3 weeks
|
Reporting & Delivery of Culture Supernatant/Cell Pellets (Go/No-Go) |
||
| Purification and Downstream Process Development |
|
2+ Weeks
|
|
Characterization and Analysis
|
|
2+ Weeks
|
|
Formulation
|
|
2+ Weeks
|
Reporting and Delivery of Purified Protein |
Additional Services:
Stable Cell Line Development
Stable cell line development offers a robust solution for sustained and reliable protein expression in mammalian cells which is necessary for therapeutics, large-scale manufacturing, or other applications where consistent expression over multiple generations is critical. Stable cell lines can often provide higher per volume protein expression levels compared to transient gene expression. We offer a complete solution to provide you with fully documented and characterized cell banks for seamless transfer to a manufacturing facility for further production. Our proprietary Bioneer BION-CHO 6.0 System™ ensures high titers and consistent protein expression levels across generations, validated across various protein modalities. With innovative technologies and a multidisciplinary approach, we are well-equipped to tackle specialized projects.
- Systems: CHO-DG44, CHO-S, BION-CHO 6.0 System™, or your preferred system
- Stable Pools in as little as 8 weeks
- Monoclonal Cell Lines in as little as 12 weeks
- Fully characterized cell line
- Research Cell Bank (RCB) tested for sterility and mycoplasma
- Monoclonality verified and documented
- Stability over 60 generations verified and documented
- BSE/TSE statement provided
- Documented cell line history
- Yields up to 6 g/L
- Royalty-free
BION-CHO 6.0 System™
The Bioneer BION-CHO 6.0 System™ outperforms commercially available systems. By utilizing our proprietary vectors specifically designed for use in CHO-DG44 cells, we achieve a significant increase in expression levels, which can be further enhanced through MTX amplification and media optimization.
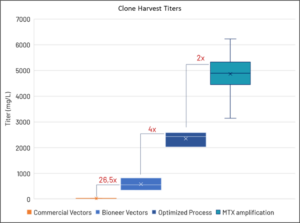
We have successfully demonstrated the expression of various protein modalities, including enzymes, monoclonal antibodies (mAbs), and Bispecifics, maintaining stable expression over 60 generations, even in cells subjected to MTX amplification. With demonstrated titers reaching up to 6 g/L for mAb expression, the BION-CHO 6.0 System™ is the premium choice for a system.
Workflow at Bioneer
Leveraging our expertise across diverse protein types, we provide early insights into the expression of your protein and mitigate risk through a flexible step-wise project approach with Go/No-Go decision points.
Process Step |
Our approach |
Time |
|---|---|---|
|
Gene Design and Plasmid Preparation
|
|
3 – 6 weeks
|
|
Transfection and Generation of Stable Pools
|
|
3 weeks
|
| Optional MTX Amplification |
|
3 weeks |
| Optional Fed-batch Shake-Flask Production |
|
2 – 3 weeks |
Reporting & Transfer of Stable Pools, Supernatants, or Purified Proteins (Go/No-Go) |
||
| Single Cell Cloning, Expansion, and Screening |
|
4 Weeks
|
| Production Models and Selection of Top Clones |
|
3 Weeks
|
| RCB Banking of Top Clones |
|
2 Weeks
|
Reporting & Transfer of Stable Pools, Supernatants, or Purified Proteins (Go/No-Go) |
||
| Expression and Genetic Stability Study of Top Clones |
|
13 weeks |
| Upstream Process Development |
|
3+ weeks |
| Purification and Downstream Process Development |
|
2+ weeks |
| Characterization and Analysis |
|
2+ weeks |
| Formulation |
|
2+ weeks |
Reporting and Delivery of Purified Protein |
Additional Services:
- Cell Line Engineering
- Glyco-engineered cell lines
Upstream Process Development
Optimization of cultivation conditions is not just important, but critical for achieving maximum yield in protein expression. By adjusting factors such as temperature, pH, and nutrient composition, we can tailor a cultivation process specifically designed to maximize production in your chosen mammalian cell line.

Beginning with small-scale experiments in tubes and shake-flasks, we methodically identify the most favorable expression conditions and can further test scalability utilizing single-use bioreactors. Whether you are seeking to develop a new process, optimize an existing one, conduct confirmation runs, or engage in pre-clinical production, our expertise and resources are at your disposal.
- Fed-batch Production
- Increase yields up to 2-fold
- 2x 1 L Single Use Bioreactors
- Media and process optimization
Purification and Downstream Process Development
We specialize in developing customized downstream processes, drawing upon our profound understanding of the inherent complexities in protein purification and the unique characteristics of each protein. We can swiftly provide materials using standardized methods such as affinity chromatography or devise intricate multistep processes that ensure purity levels surpassing 99%.

With experience in developing processes for cGMP manufacturing, our processes are carefully designed for seamless transfer to manufacturing facilities. Whether you require rapid material, full-scale process development, or optimization of existing procedures, our team of experts is fully equipped to assist you every step of the way.
- Tagged and Non-tagged Methods
- Upscaling and Tangential Flow Filtration
- Process Development & Troubleshooting
- mg to g scale
Variety of chromatographic techniques available
- Affinity chromatography (Protein A, L, IMAC, etc.)
- Ion exchange chromatography
- Size exclusion chromatography
- Mixed mode chromatography
- Hydrophobic interaction chromatography
- Endotoxin removal chromatography
- More!
General Workflow
While every protein is different, our workflows are designed to optimize speed, resolution, capacity and yield. The following outlines the general steps in a downstream process.
Process Step |
Example techniques |
|
|---|---|---|
| Preparation, Extraction, Clarification |
|
|
|
Capture (Isolate, concentrate, and stabilize) |
|
|
| Intermediate Purification
(Remove Bulk Impurities) |
|
|
| Polishing
(Achieve final high level purity) |
|
|
| Finalizing/ Formulation
(final preparation) |
|
Characterization & Analysis
We specialize in offering customizable characterization packages for protein analysis, utilizing a diverse array of techniques to meticulously examine protein properties. Through close collaboration with you, we tailor a plan to explore various facets of your protein, including purity, identity, structure, aggregation and stability.

Additionally, our cross-disciplinary approach enables us to provide further insights into your protein’s functionality by leveraging our in vitro modelling, histology, or formulation services. Whether you’re immersed in fundamental research, assay production, or biopharmaceutical development, our comprehensive characterization packages deliver invaluable insights to propel your projects forward.
Select from a variety of techniques
- Gel electrophoresis (SDS-PAGE, native PAGE, IEF)
- Western blotting
- Capillary electrophoresis (CGE and cIEF)
- Bradford and BCA protein quantification
- Quantitative amino acid analysis (outsourced)
- UV spectrophotometry
- UHPLC (SEC, IEX, HIC, RP chromatography)
- ELISA
- Enzyme assays
- Endotoxin measurement
- Host cell protein analysis (CHO and E. coli HCP, Cygnus kit)
- Host cell DNA (qPCR method)
- Mass spectrometry (outsourced)
- Circular Dichroism
- Dynamic light scattering
- Infrared spectroscopy
- Thioflavin T Fluorescence
- DSC and ITC
- Mass Photometry (Refeyn TwoMP)
Additional Services:
For further information
please contact:
Ryan Jeffrey Polito
Business Development Manager


