Early Stage Drug Candidates
Bioneer has decades of experience in all aspects of developing and producing drug candidates for non-GMP use.
We cover recombinant proteins, peptides and small molecules and assist companies in their earliest phases producing drug candidate to formulation development.

Recombinant Proteins
We help you get from your idea to your first protein and product. We are experts in all aspects of expression, purification, characterization, formulation and small-scale non-GMP manufacturing of recombinant proteins.
Together with our partners, we continue to expand our comprehensive service solutions to serve as your end-to-end recombinant protein partner through-out discovery and early development of your drug candidates. We are equipped to transfer your cell bank, your process and associated documentation to CMO’s of your preference for larger scale cGMP manufacturing.
We have 30+ years experience in recombinant protein development and have expressed over 300 proteins, including Membrane Proteins, Cytokines, Hormones, Antibodies (mAbs/Fabs), Growth Factors, Fusion Proteins, Enzymes and Serpins.
Bioneer Recombinant Service Solution
Bioneer offers a complete non-GMP service solution for recombinant protein expression and production, from gene to first protein, utilizing microbial and mammalian platforms.
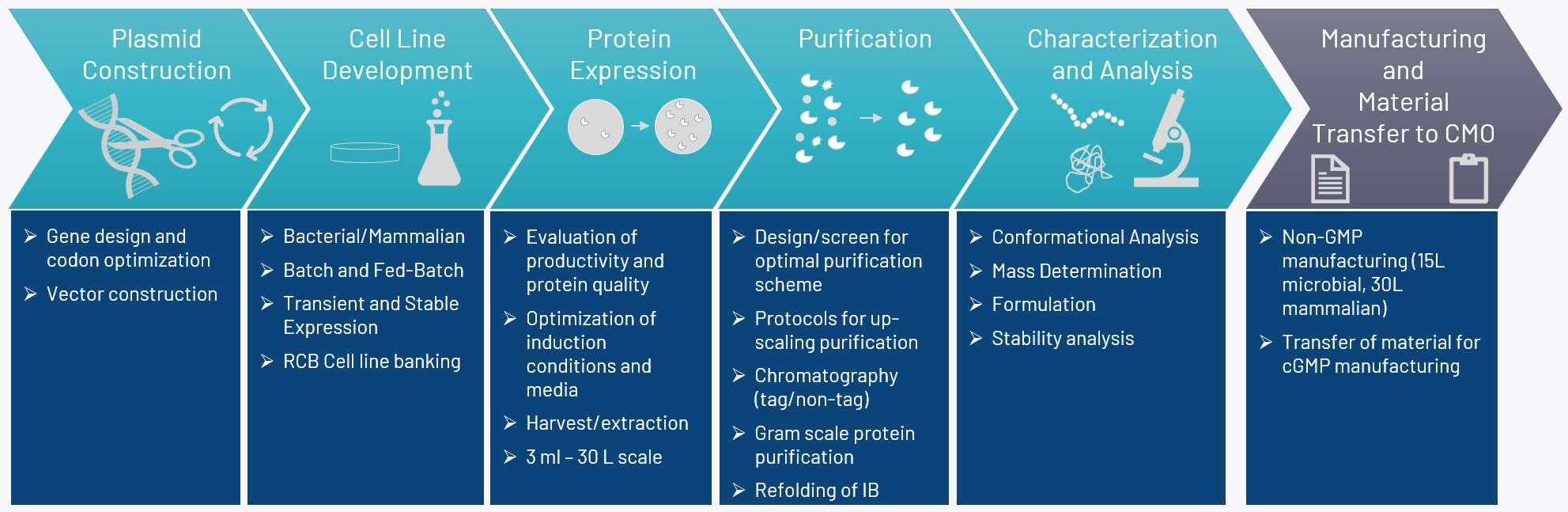
Feasibility studies
- Expression Systems: Working closely with you, we identify the optimal expression system for your protein using bacterial and/or mammalian expression systems including Escherichia coli, Lactococcus lactis, Chinese hamster ovary cells (CHO) and Human embryonic kidney cells 293 (HEK293).
- Gene Design and Expression Constructs: Synthetic codon optimized genes are designed and cloned into the selected expression vectors. Expression constructs are introduced into the selected host system.
- Protein Expression: Rapid expression of your protein in mg quantities can be done in less than 5 weeks using the selected expression system. Evaluation of expression is analyzed by SDS-PAGE or western blot analysis. Larger quantities can be produced in a non-GMP environment at up to 15 L scale using bacterial expression platforms or up to 30 L using mammalian cell lines.
- Research Cell Banks: We generate research cell banks and perform QC characterization, e.g. by DNA sequencing. Cell banks can subsequently be transferred to CMOs for cell banking according to cGMP guidelines.
Upstream Process Development
- Fermentation: We develop processes for recombinant protein production in flasks, batch and fed-batch conditions in bioreactors up to 15 L scale. We have equipment for clarification of fermentation broth by centrifugation and tangential flow filtration. Intracellular proteins are extracted using high-pressure homogenization. Periplasmic proteins are extracted from E. coli using an osmotic shock procedure.
- Mammalian Cell Protein Production and Cell Line Development: We develop processes for recombinant protein production in mammalian cells using either transient or stable expression up to 30 L scale. Stable cell lines for research, preclinical development, or transfer to CMO facility can be generated from CHO host cells. Stable pools can be developed in as little as 9 weeks and we can screen and select for high producing, single cell clones. Proof of monoclonality, RCB banking, and cell line characterization to assess expression and genetic stability of top clones can also be performed.
DTU National Biologics Facility
- Bioneer has partnered with the commercial arm of the DTU National Biologics Facility, which brings expert knowledge of CHO mechanisms and hands-on experience in CHO cell line development and recombinant protein production in mammalian cells. Read more about NBF here.
Downstream Process Development
- Purification: Our facility is equipped with several ÄKTA purification stations and we offer a variety of chromatographic column-based purifications methods based on affinity, ion-exchange, hydrophobic interaction, mixed mode and gel filtration. Purification in gram scale is possible.
- Refolding of Proteins: Recombinant proteins produced in inclusion bodies (E. coli expression) are solubilized and refolded by e.g. dilution.
- Characterization and Analytics: We characterize the final recombinant protein with respect to quantity and quality using techniques such as:
- Electrophoresis (SDS-PAGE, native PAGE, IEF)
- Capillary electrophoresis (CGE and cIEF)
- Western blotting
- Activity assays
- UV spectrophotometry
- HPLC (SEC, IEX and reversed phase chromatography)
- Endotoxin measurement
- Host cell protein (HCP) analysis
- Host cell DNA analysis
- Circular dichroism
- Dynamic light scattering
- Mass photometry (Refeyn TwoMP)
- MS analysis for protein identification, MW determination, peptide mapping, HCP analysis etc. (outsourced to third party)
- Glycoprofiling
Technology Transfer
- Tech Transfer to CMO for cGMP manufacturing: In close collaboration with our clients, we prepare a tech transfer package securing smooth transfer to the selected CMO responsible for cGMP manufacturing.
Formulation
- We help you design and perform the critical experiments, which are necessary for creating a stable protein formulated according to regulatory requirements.
For further information, please contact Head of Department Søren Madsen by phone or email.
Feasibility studies
- Expression Systems: Working closely with you, we identify the optimal expression system for your protein using bacterial and/or mammalian expression systems including Escherichia coli, Lactococcus lactis, Chinese hamster ovary cells (CHO) and Human embryonic kidney cells 293 (HEK293).
- Gene Design and Expression Constructs: Synthetic codon optimized genes are designed and cloned into the selected expression vectors. Expression constructs are introduced into the selected host system.
- Protein Expression: Rapid expression of your protein in mg quantities can be done in less than 5 weeks using the selected expression system. Evaluation of expression is analysed by SDS-PAGE or western blot analysis. Larger quantities can be produced in a non-GMP environment at up to 15 L scale using bacterial expression platforms or up to 16 L using mammalian cell lines.
- Research Cell Banks: We generate research cell banks and perform QC characterization, e.g. by DNA sequencing. Cell banks can subsequently be transferred to CMOs for cell banking according to cGMP guidelines.
Upstream Process Development
- Fermentation: We develop processes for recombinant protein production in flasks, batch and fed-batch conditions in bioreactors up to 15 L scale. We have equipment for clarification of fermentation broth by centrifugation and tangential flow filtration. Intracellular proteins are extracted using high-pressure homogenization. Periplasmic proteins are extracted from coli using an osmotic shock procedure.
Downstream Process Development
- Purification: Our facility is equipped with several ÄKTA purification stations and we offer a variety of chromatographic column-based purifications methods based on affinity, ion-exchange, hydrophobic interaction, mixed mode and gel filtration. Purification in gram scale is possible.
- Refolding of Proteins: Recombinant proteins present in inclusion bodies ( coli expression) are solubilized and refolded by dilution into appropriate buffer solutions.
- Characterization and Analytics: We characterize the final recombinant protein with respect to quantity and quality using techniques such as:
- Electrophoresis (SDS-PAGE, native PAGE, IEF)
- Bradford and BCA protein quantification
- UV spectrophotometry
- HPLC (SEC, IEX and reversed phase chromatography)
- Endotoxin measurement
- Host cell protein analysis
- Host cell DNA analysis
- Circular dichroism
- Dynamic light scattering
- Yield and quality can be analyzed by SDS-PAGE, Western blotting or activity assays.
Technology transfer
- Tech Transfer to CMO for cGMP Manufacturing: In close collaboration with our clients, we prepare a tech transfer package securing smooth transfer to your CMO responsible for cGMP manufacturing.
Formulation
- We help you design and perform the critical experiments, which are necessary for creating a stable protein formulated according to regulatory requirements.
For further information, please contact Head of Department Søren Madsen by phone or email.
Peptides
Bioneer develop pre-formulation strategies and we design and perform the critical experiments necessary for creating stable peptides formulated according to regulatory requirements. We collaborate with private partners having a proven track record in bringing biologics through the pre-clinical and clinical phases to assist companies all the way.
Peptide formulation services
We carry out customized pre-formulation projects with peptide drug candidates including characterization of physicochemical properties, and determination of the chemical and physical stability to support a rational formulation development process.
Based on physicochemical data different formulation compositions can be designed in collaboration with the client and characterized in terms of chemical and physical stability. Furthermore, we offer services in our gastrointestinal models, such as the Dynamic Gastric Model and in vitro lipolysis model to support the development of oral peptide formulations. We can also assist in the development and characterization of freeze-dried peptide formulations and we have the capacity to produce technical batches of freeze-dried formulations.
Physicochemical characterization of peptides can include
- Visual inspection (visible particles)
- pH measurements
- Characterization of subvisible particles by Dynamic Light Scattering (DLS) measurements
- Dissolution time/reconstitution time
- Determination of the osmolality of peptide formulations by freezing point depression measurements
- Quantification of peptide concentrations in small volumes by UV-absorbance measurements in Nanodrop equipment
- Quantification of peptides by means of HPLC-UV, UPLC-UV and LC-MS (QQQ)
- Small Angle X-ray Scattering (SAXS)
We can design formulation projects and experiments to enable screening of multiple variables of your choice (e.g. different excipients, formulation compositions, pH-values, salt concentrations etc.).
Stability analysis
We determine chemical stability of peptides based on forced degradation and long-term stability studies supported by HPLC-UV, UPLC-UV and LC-MS quantification.
We determine physical stability of peptides based on e.g. agitation/shaking and long-term stability studies supported e.g. by HPLC-SEC. Furthermore, we carry out fibrillation studies using Thioflavin-T Assays and aggregation studies by DLS.
For further information on immunology, please contact Department Head Anette Müllertz by phone or email, or Analytical Scientist Tania Lind by phone or email.
Peptide formulation services
We carry out customized pre-formulation projects with peptide drug candidates including characterization of physicochemical properties, and determination of the chemical and physical stability to support a rational formulation development process.
Based on physicochemical data different formulation compositions can be designed in collaboration with the client and characterized in terms of chemical and physical stability. Furthermore, we offer services in our gastrointestinal models, such as the Dynamic Gastric Model and in vitro lipolysis model to support the development of oral peptide formulations. We can also assist in the development and characterization of freeze-dried peptide formulations and we have the capacity to produce technical batches of freeze-dried formulations.
Physicochemical characterization of peptides can include
- Visual inspection (visible particles)
- pH measurements
- Characterization of subvisible particles by Dynamic Light Scattering (DLS) measurements
- Dissolution time/reconstitution time
- Determination of the osmolality of peptide formulations by freezing point depression measurements
- Quantification of peptide concentrations in small volumes by UV-absorbance measurements in Nanodrop equipment
- Quantification of peptides by means of HPLC-UV, UPLC-UV and LC-MS (QQQ)
- Small Angle X-ray Scattering (SAXS)
We can design formulation projects and experiments to enable screening of multiple variables of your choice (e.g. different excipients, formulation compositions, pH-values, salt concentrations etc.).
Stability analysis
We determine chemical stability of peptides based on forced degradation and long-term stability studies supported by HPLC-UV, UPLC-UV and LC-MS quantification.
We determine physical stability of peptides based on e.g. agitation/shaking and long-term stability studies supported e.g. by HPLC-SEC. Furthermore, we carry out fibrillation studies using Thioflavin-T Assays and aggregation studies by DLS.
For further information on immunology, please contact Department Head Anette Müllertz by phone or email, or Analytical Scientist Tania Lind by phone or email.
Small Molecules
We specialize in pre-formulation and strategic formulation of small molecules.
We specialize in pre-formulation and strategic formulation of small molecules.
Pre-formulation & Physicochemical Characterization
We determine the right drug delivery strategy for a molecule investigating factors such as solubility, intrinsic dissolution rate, supersaturation propensity, solid state properties and stability.
Formulation Development
We develop conventional pharmaceutical formulations as well as enabling drug delivery systems for problem drugs. We have all needed equipment to develop e.g. coated tablets and liquid filled capsules.
In vitro evaluation of formulations
Formulations are evaluated and characterized by state-of-the-art in vitro characterization tools simulating the in vivo situation as close as possible.
For further information, please contact Department Head Anette Müllertz by phone or email.
We specialize in pre-formulation and strategic formulation of small molecules.
Pre-formulation & Physicochemical Characterization
We determine the right drug delivery strategy for a molecule investigating factors such as solubility, intrinsic dissolution rate, supersaturation propensity, solid state properties and stability.
Formulation Development
We develop conventional pharmaceutical formulations as well as enabling drug delivery systems for problem drugs. We have all needed equipment to develop e.g. coated tablets and liquid filled capsules.
In vitro evaluation of formulations
Formulations are evaluated and characterized by state-of-the-art in vitro characterization tools simulating the in vivo situation as close as possible.
For further information, please contact Department Head Anette Müllertz by phone or email.
